Research
1) Next-generation chemical proteomic platforms
2) New paradigms in metabolic signaling and disease
3) Targeting Transcription Factors with Next Generation Biologics
Active Areas of Research
Biological systems are inherently heterogeneous and dynamic, both at the molecular level (e.g., encoded proteins existing in distinct posttranslational modification states) and the cellular level (e.g., organization of biomolecules to distinct regions of a cell or distinct cells within a tissue). To understand endogenous regulatory mechanisms in these systems under basal or diseased states, we must be able to quantify the functional states of biomolecules in native environments across scales of space and time. The Moellering lab is focused on developing new chemical probes and complementary proteomic technologies to enable quantitative measurements in the proteome in native contexts currently out of reach – ranging from sub-cellular complexes, single cells, tissues to live animals. We use tools to discover and unravel new signaling mechanisms in the proteome, which can serve as the basis for diagnostic and therapeutic development. In parallel, we apply our expertise in synthesis and protein chemistry to develop new types of chemical probes and prototype therapeutics with a focus on targeting so-called ‘undruggable’ targets in disease. This work spans the design and development of small molecules, peptidomimetics and proteomimetics. The long-term goal of our research program is to enact a paradigm shift in the way we study and target disease-relevant signals in human diseases like cancer, immunological disorders and degenerative diseases.
- Chemical proteomic probes and platforms for interrogation of protein function, interactions and crosstalk in native biological environments ranging from single cells to live animals.
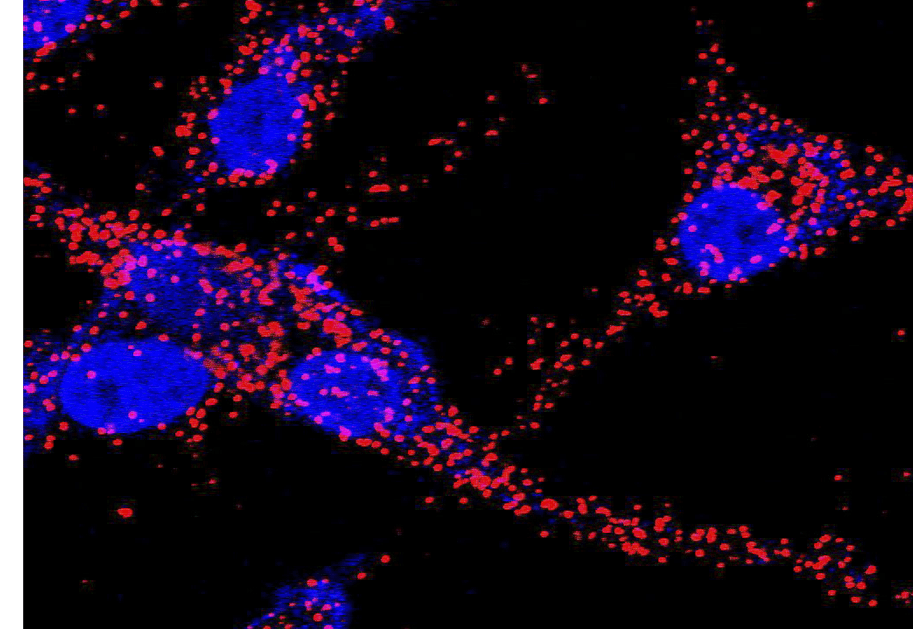
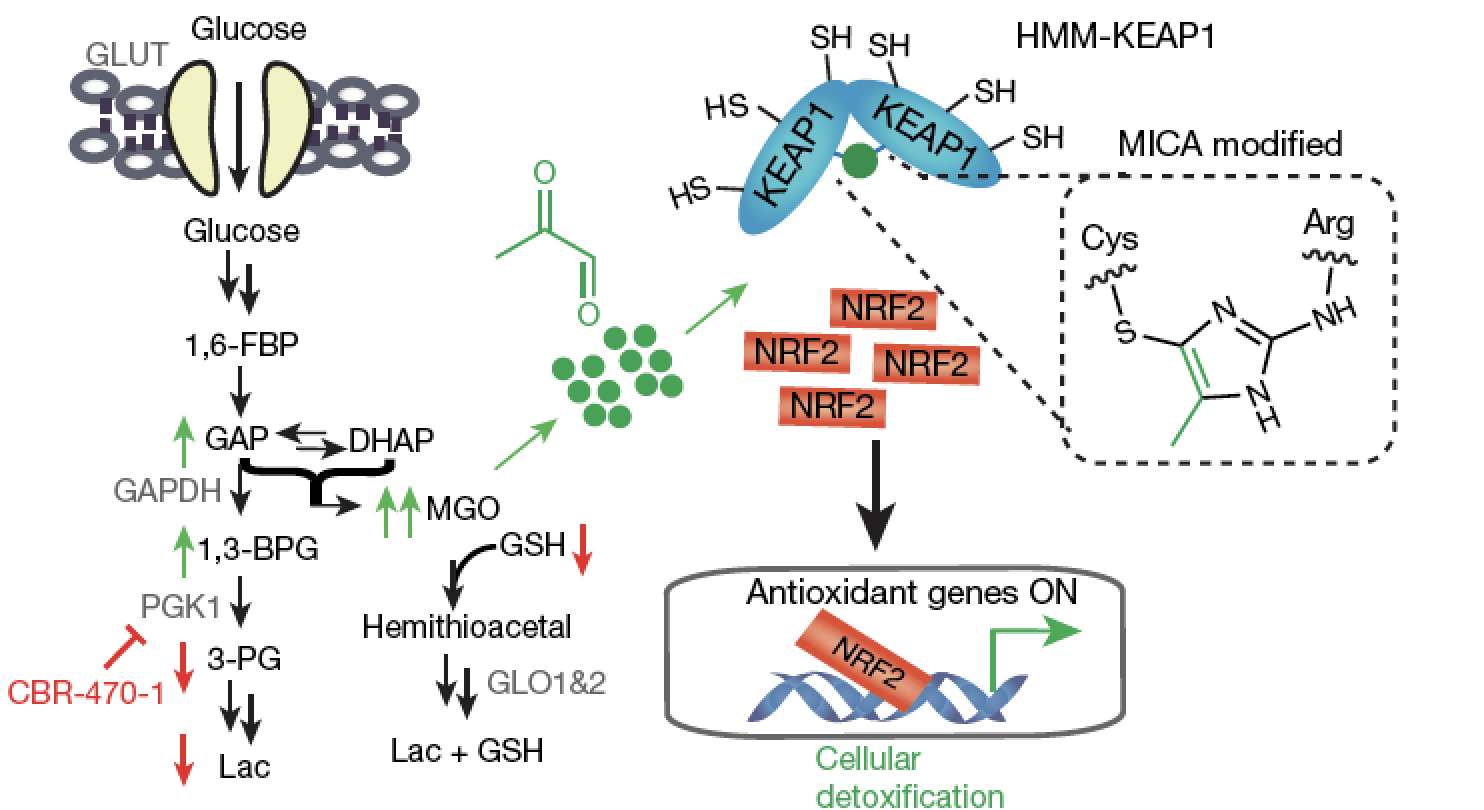
- We are interested in mapping regulatory interactions in normal and disease-associated metabolic programs. These efforts include understanding how protein-protein and protein-metabolite interactions, as well as their spatial compartmentalization in cells and tissues, shape the flow of metabolism. We are particularly interested in intrinsically reactive metabolite-mediated posttranslational modifications (rmPTMs) and their ability to serve as metabolic signals. A goal of these research projects is to identify regulatory nodes and coupled small molecule probes that can control them for intervention in diseases like cancer, diabetes, inflammation and aging.
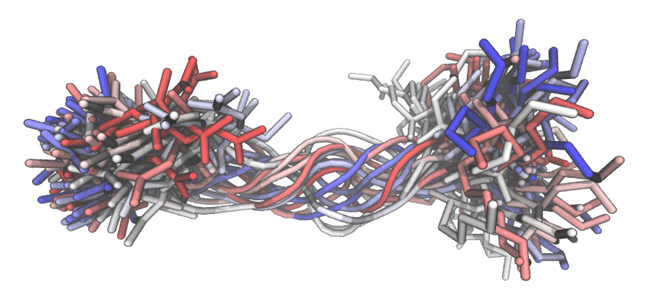
- Developing and applying new chemical strategies to control protein structure for the development of synthetic peptide and protein therapeutics.
